Home
→
Sharing Info
→ Stelara offers patients a new treatment option
Stelara offers patients a new treatment option
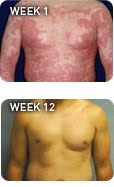
Stelara, a new drug for treating moderate to severe psoriasis in adults, was approved today by the U.S. Food and Drug Administration. Known by the generic name ustekinumab, the drug is injected in the skin, and works by blocking part of the body's immune system.
Stelara is a "very significant medication," said Dr. Craig Leonardi, a dermatologist in St. Louis, Mo. who served as a lead investigator in clinical trials for the drug. Leonardi believes Stelara is particularly effective for patients who've seen little or no response to treatments with other medications.
"Another unique feature of this drug," Leonardi added, "was when patients were doing well, they were treated to a 'drug holiday' to see how fast it came back." When Stelara was withdrawn, psoriasis symptoms were slow to return.
It's not a cure," Leonardi said, "but psoriasis did not come back with a vengeance."
The drug blocks two proteins—interleukin 12 and interleukin 23—produced by the immune system, according to Bruce Bebo, Jr., director of research and medical programs for the National Psoriasis Foundation. The proteins are believed to play an important role in the development of psoriasis and other inflammatory diseases.
In psoriasis and psoriatic arthritis, the body's immune system behaves as though it is under attack, overproducing parts of the body's chemical arsenal.
In June 2008, Dan Farrington, now chair-elect of the National Psoriasis Foundation Board of Trustees, and Foundation volunteer Ellen Clements testified on behalf of the drug. Both shared their experiences living with psoriasis and emphasized that a "one-size-fits-all" treatment approach does not work. At the hearing's close, the committee voted unanimously to recommend that the FDA approve Stelara to treat moderate to severe psoriasis.
Stelara "adds a lot to our arsenal," said Bebo. "We're always happy when there is a new tool for treating psoriasis. It's a pretty big new step."
A study in the British medical journal The Lancet earlier this year noted that as many as 42 percent of patients treated in a clinical trial with Stelara saw the severity of their psoriasis reduced by 90 percent. "Side effects in the clinical trial were generally mild," Bebo noted.
Stelara was approved in Canada last December for treating moderate to severe psoriasis in adults, following clinical trials that involved more than 3,000 patients. It has also been approved for use in Europe.
Tags:
Sharing Info
Subscribe to:
Post Comments (Atom)
Search This Blog
Popular Posts
-
Kebiasaannya apabila berjumpa doktor kulit, pesakit eczema dan psoriasis akan diberi berbotol-botol krim pelembap aqueous untuk menguran...
-
Typically, psoriasis is reddish as well as pinkish areas connected with solid, increased, and/or dry out pores and skin. The most frequent ...
-
Both psoriasis and eczema are chronic skin diseases that cause red, scaly skin rashes. Though they are quite similar, the range of symptoms ...
-
Benefits of Amla Powder Natural Astringent When mixed with water, amla powder becomes a natural astringent for the face and hair, acc...
-
Psoriasis kulit kepala adalah penyakit kulit kronik yang berlaku apabila sel-sel anda dengan cepat membiak dan mengumpul pada permukaan...


“I read these posts and loved to share my knowledge using natural herbal formula and a lifestyle change.
ReplyDeleteAs an ICU nurse with an amazing 38years old career, I know what a medical miracle is. For no reason, I have never liked herbal medicines until I witnessed a confirmed HIV positive patient test result reversed to HIV negative after conducting more tests on him which all came back HIV negative.
Back then many experts who saw the test results gave different suggestions to their best understanding and why it was possible or impossible. But to me, it was nothing more than a medical miracle.
I scrutinized him and found out he has been cured with herbal medicine which a physician have prepared for him.
I also suffered from GENITAL HERPES back then which was the primary reason that has made me remain single and my biggest secret that has made me understand life in the best way I should.
Through this abovementioned person who had been cured of HIV, I contacted this physician who permanently cured me GENITAL HERPES with herbal medicine and also cured a PSORIASIS patient with herbal medicine through me, and in all these tribulations and recovery phases, we conquered.
The good news is that this physician is available on:
drutuherbalcure@gmail.com
+2349072733661